 |
Osteoallograft® Orthomix® is natural, real bone allograft designed specifically for veterinary use. It consists of osteoinductive Demineralized Bone Matrix (DBM) and osteoconductive cancellous bone chips. Using bone allograft allows you to avoid autograft procurement and associated morbidity risks. Also, because it not only provides osteoconductive scaffold but also native, osteoinductive growth factors (BMPs), you will achieve faster and stronger bone healing compared with using bone substitutes. [1]
The demineralization of allograft during tissue processing allows for immediate access to the growth factors (BMPs) inherent in natural bone when it is placed into the surgery site. This results in an immediate beginning of the healing process and helps to make allograft as effective as autograft. [2-4]
Osteoallograft Orthomix is professional-grade allograft:
• Processed aseptically meeting USP guidelines for sterility
• Acellular and processed by methods that reduce immunogenicity, which eliminates concerns about immune reactions and the need for any type of patient matching.
• Our production practices are GMP and Good Tissue Practices compliant and modeled after human tissue banking standards
• Our stringent Quality Assurance Program provides confidence and consistency in our products
Indications
Use Osteoallograft® Orthomix® for:
• Fracture repair
• Mal- or non-union cases
• Arthrodesis procedures
• Bone loss
• TTAs and TPLOs [2,5]
• Any other application where bone graft is needed
Veterinarian & Patient Benefits
Why graft:
• Bone will heal faster when voids are filled with bone graft, because it provides osteoconductive scaffold for host bone to grow on and native, osteoinductive BMPs that attract osteoblasts to the site. Even when there are no voids, bone graft provides these same advantages. [2,6,7]
• Faster healing not only gets your patients back to normal activity faster it also increases the chances of a successful healing outcome [2,6,7]
Why use allograft:
• Reduces your OR time and cost, because it allows you to skip autograft harvesting [8]
• Eliminates morbidity risks associated with the collection of bone autograft. Studies show a morbidity rate of over 25% in humans [9]
• In many cases, allows you to use more bone graft than you can procure from the patient. Allograft can also be used to augment insufficient quantities of autograft.
• Studies show that allografts are as effective as autograft in bone healing [2-4]
• Achieves faster and stronger bone healing than bone substitutes due to osteoinductive growth factors (BMPs) inherent in natural bone.[1] To see Grafting Options Comparison Chart, click here.
Available Product Types

Product Name:Orthomix® – Fine*
*Standard and Ultra-fine Orthomix available through VTS
Particle Size: Demineralized Bone Matrix with cancellous chips < 2.3 mm
Preservation: Freeze-Dried*
*Frozen orthomix available through VTS
Volumes Available: Canine 1cc, 2cc*
*More sizes available through VTS
Images
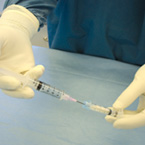
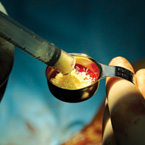
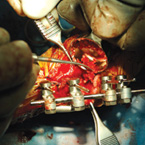
| Osteoallograft™ Orthomix™ is easy to use: | |||
 |
 |
 |
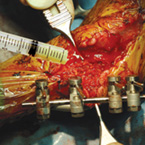 |
| Rehydrate graft with saline. | Mix Osteoallograft® Orthomix® with patient blood or bone marrow. | Apply to surgical site. | When working in a vascular site, grafts can be applied directly out of the syringe. |
About VTS
Veterinary Transplant Services, Inc. (VTS) is the world’s first animal tissue bank. VTS provides veterinarians with animal bone and soft tissue allografts for animal use. Using professionally-procured bone allograft saves them from having to procure the patient’s own bone when bone material is needed. This decreases their OR time & cost and spares the patient a second operative site.
Orthopedic surgeons in human medicine have been confidently using bone allografts for decades. As a veterinary version of a human tissue bank, VTS brings this significant medical advancement to veterinarians and are proud of having been doing so successfully since 1996.
Besides the most commonly used Osteoallograft® Orthomix®, cancellous blocks, bone sections, and whole bones are also available for spinal fusions, severe fractures, and limb sparing procedures.
Support Materials
Osteoallograft Orthomix Brochure (PDF: 738 KB)
Osteoallograft Orthomix Case Study with Shaft Section (PDF: 578 KB)
Frequently Asked Questions (PDF: 111 KB)
Literature List with Excerpts (PDF: 173 KB)
A Word from the Director (PDF: 233 KB)
Clinical References:
- Griffon DJ, Dunlop DG, Howie CR, Gilchrist T, Salter DM, Healy DM. Early dissolution of a morsellised impacted silicate-free bioactive glass in metaphyseal defects. J Biomed Mater Res (Applied Biomater). 58(6):638-644, 2001.
- Hoffer M, Griffon D, Schaeffer D, Johnson A, Thomas M. Clinical applications of demineralized bone matrix: A retrospective and case-matched study of 75 dogs. Vet Surg. 37:639-647, 2008.
- Samartzis D, Shen FH, Matthews DK, Yoon ST, Goldberg EJ, An HS. Comparison of allograft to autograft in multilevel anterior cervical discectomy and fusion with rigid plate fixation. Spine J. Nov-Dec 3(6): 451-9, 2003.
- Piotrowski M, Pankowski R, Luczkiewicz P, Markowicz A. A comparison of the effect of autogenous vs. frozen homogenous grafts on the healing of non-union of forearm bones. Ortop Traumatol Rehabil. 10(2):146-51, 2008.
- Lafaver S, Miller NA, Stubbs WP, Taylor RA, Boudrieau RJ. Tibial tuberosity advancement for stabilization of the canine cranial cruciate ligament-deficient stifle joint: surgical technique, early results, and complications in 101 dogs. Veterinary Surgery. 36:573-586, 2007.
- Kesemenli CC, Kapukaya A, Subasi M, Arslan H, Necmioglu S, Kayikci C. Early prophylactic autogenous bone grafting in type III open tibial fractures. Acta Orthop Belg. 70(4):327-31, 2004.
- DeVries WJ, Runyon CL, Martinez SA, Ireland WP. Effect of volume variations on osteogenic capabilities of autogenous cancellous bone graft in dogs. Am J Vet Res. Oct 57(10):1501-1505, 1996.
- St John TA, Vaccaro AR, Sah AP, Schaefer M, Berta SC, Albert TA, Hilibrand A. Physical and monetary costs associated with autogenous bone graft harvesting. Am J Orthop. Jan 32(1):18-23, 2003.
- Younger EM, Chapman MW. Morbidity at bone graft donor sites. J Orthop Trauma. 3(3):192-195, 1989.
To see more references for use of bone graft in orthopedics, click here >>
